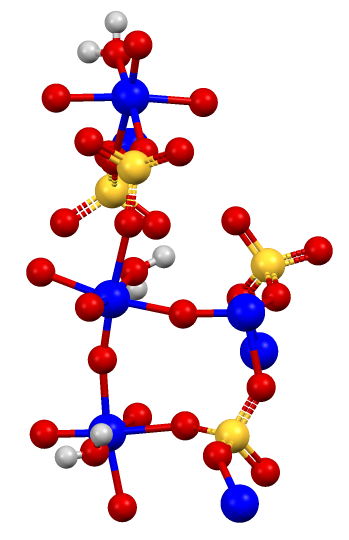
Characterization of Chemical Speciation of Titanyl Sulfate Solutions for Production of Titanium Dioxide Precipitates | Inorganic Chemistry

Supramolecular self-assembly synthesis of ordered mesoporous TiO2 from industrial TiOSO4 solution and its photocatalytic activities - ScienceDirect

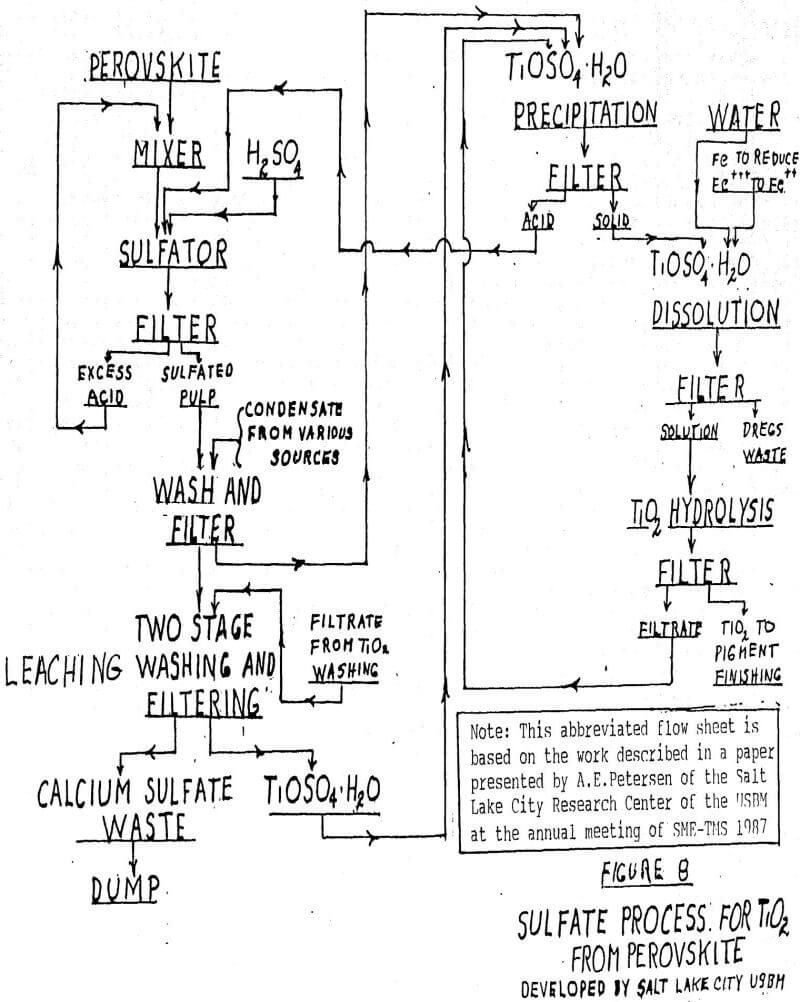
SOLVED: The flowsheet below represents the process for the production of TiO2. Sorel Slag Scrap Iron (Pure Fe) HSO4 67% by weight H2SO4 Digester Evaporator FeSO4 Air TiO2 Pure O2 Rotary Kiln

Novel Method for Preparing Amorphous Titanyl Sulfate from Hydrous Titanium Oxide | Industrial & Engineering Chemistry Research

RETRACTED ARTICLE: High-rate aluminium yolk-shell nanoparticle anode for Li-ion battery with long cycle life and ultrahigh capacity | Nature Communications
Synthesis and structural characterisation of solid titanium(IV) phosphate materials by means of X-ray absorption and NMR spectro
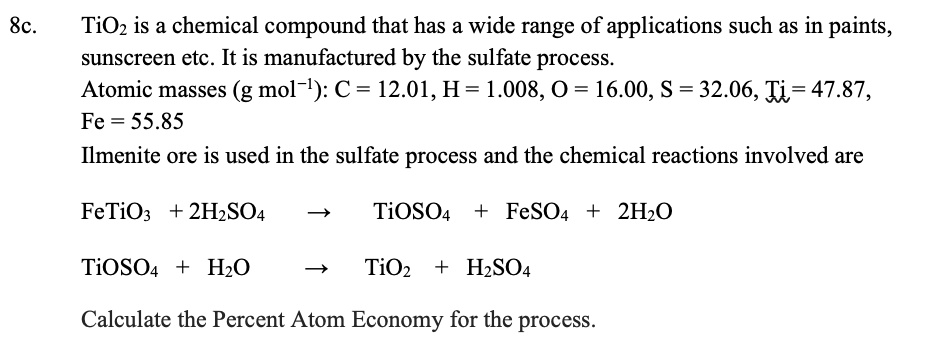
SOLVED: TiO2 is a chemical compound that has a wide range of applications such as in paints, sunscreen, etc. It is manufactured by the sulfate process. Atomic masses (g mol-1): C =

Processes | Free Full-Text | Mechanism, Thermodynamics and Kinetics of Rutile Leaching Process by Sulfuric Acid Reactions

SEM images of TiO2 products at various H2O/ TiOSO4 volume ratio of a... | Download Scientific Diagram

Cristal History 1988 – The company incorporated 1991 – The first pigment was produced 1992 – Fully operated with 4 grades at 48,000 tpa 2000 – CRISTAL. - ppt download

PDF) Effect of TiOSO 4 hydrothermal hydrolysis conditions on TiO 2 morphology and gas-phase oxidative activity | Alexander Vorontsov - Academia.edu




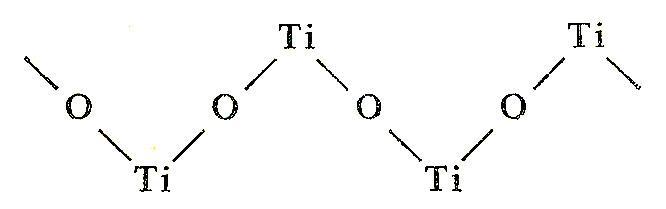


![PDF] Influence of hydrolysis in sulfate process on titania pigment producing | Semantic Scholar PDF] Influence of hydrolysis in sulfate process on titania pigment producing | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/f51fdcb711813023bae94e22833f7e56d341cbd6/3-Table2-1.png)