SOLVED: Which reaction represents an acid-base neutralization reaction? Hint: How do you identify acids and bases? O 2 NaOH + MgSO4 NaSO4 + Mg(OH)2 O HNO + KOH KNO3 + H2O O
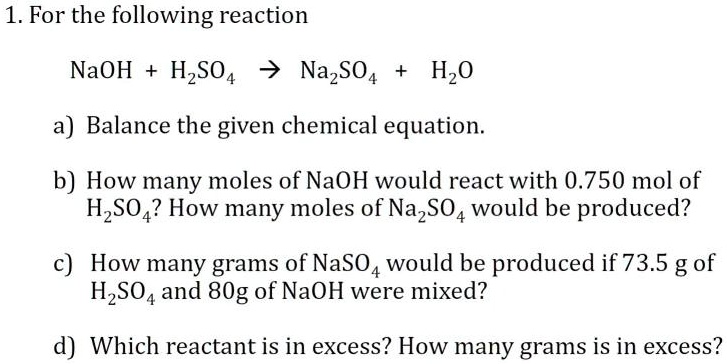
SOLVED: Texts: 1. For the following reaction: NaOH + HSO4 -> NaSO4 + H2O a) Balance the given chemical equation. b) How many moles of NaOH would react with 0.750 mol of

H Balance the following reactions NaOH + H2SO4 → Na2SO4+H2O 7 CuSO4 + NaOH → Cu(OH)2 + Na2SO4 Fe + H2O → Fe3O4+H2 4. NH4Cl + Ca(OH)2 CaCl2 + H2O + NH3 6. Fe + Cl2 → FeCl3 3. Fe
![Sodium sulfate decahydrate [Na2SO4.10H2O] (Glauber's salt) Molecular Weight Calculation - Laboratory Notes Sodium sulfate decahydrate [Na2SO4.10H2O] (Glauber's salt) Molecular Weight Calculation - Laboratory Notes](https://www.laboratorynotes.com/wp-content/uploads/2022/11/sodium-sulfate-decahydrate-molecular-weight-calculation-300x191.jpg)
Sodium sulfate decahydrate [Na2SO4.10H2O] (Glauber's salt) Molecular Weight Calculation - Laboratory Notes

Using this balanced equation: 2 NaOH + H2SO4 —> H2O + Na2SO4 How many grams of sodium sulfate will be - brainly.com