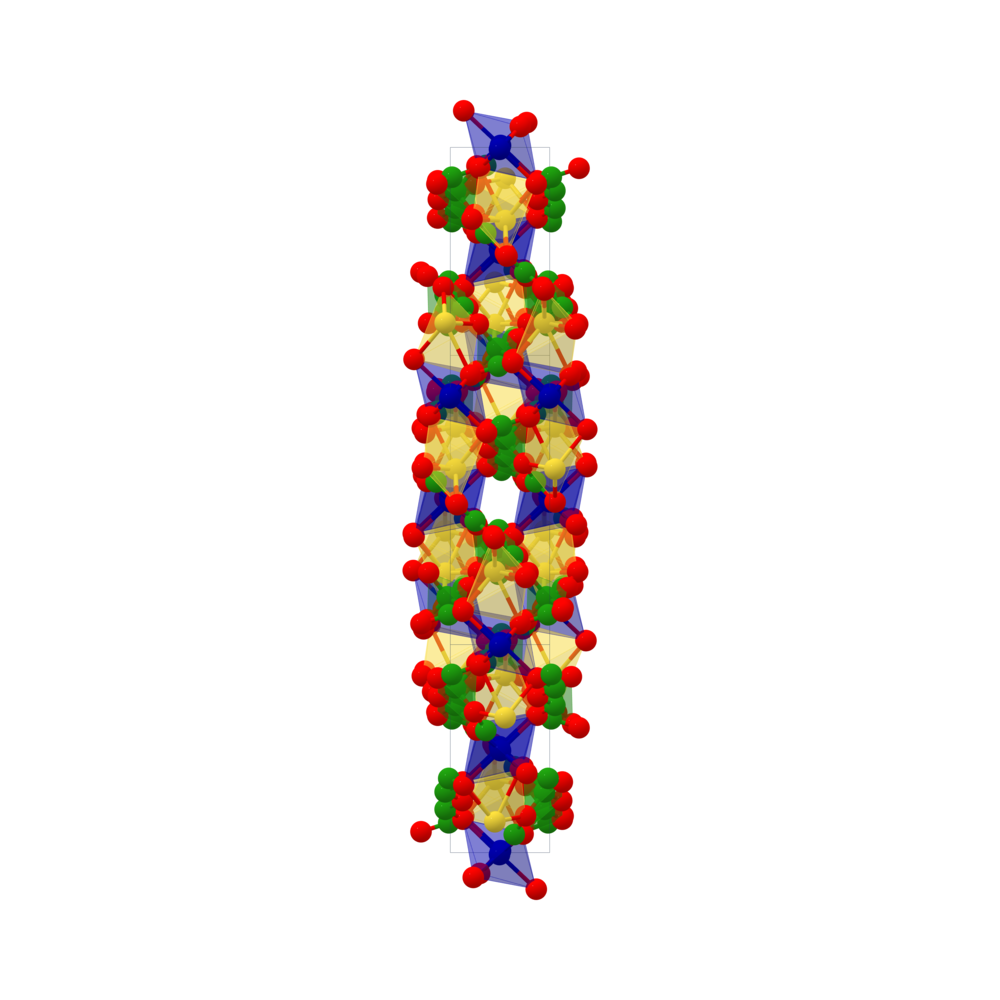
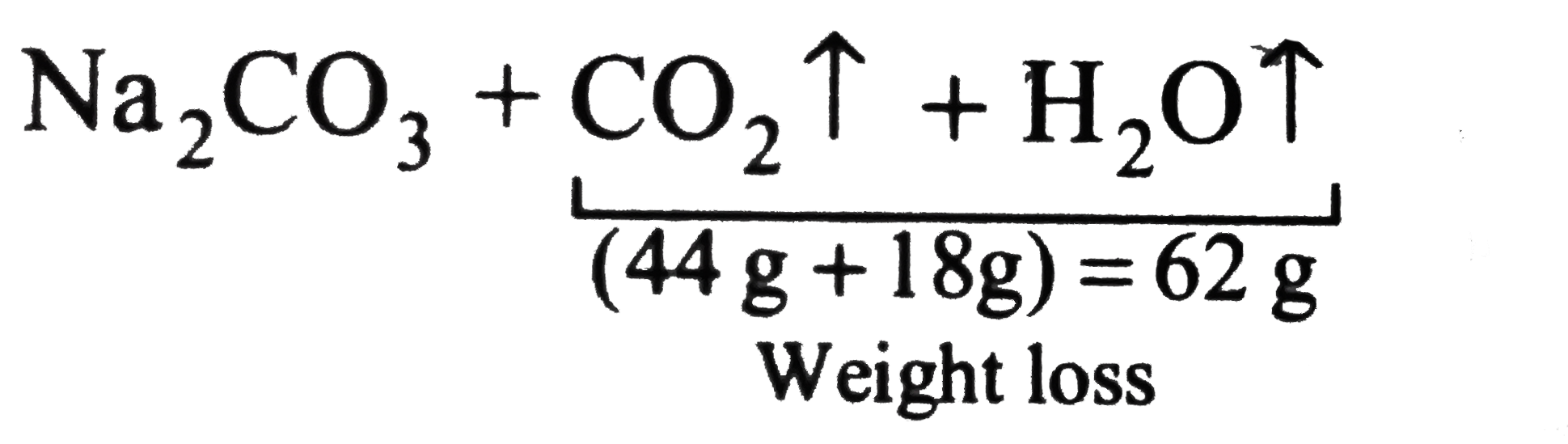Phase Diagrams of Na2CO3–CO(NH2)2–H2O2–H2O System at 0 °C and 25 °C and the Production of Urea Peroxide and Sodium Percarbonate | Journal of Chemical & Engineering Data

Water content diagram of the quaternary system K + // H 2 PO 4 2− , SO... | Download Scientific Diagram
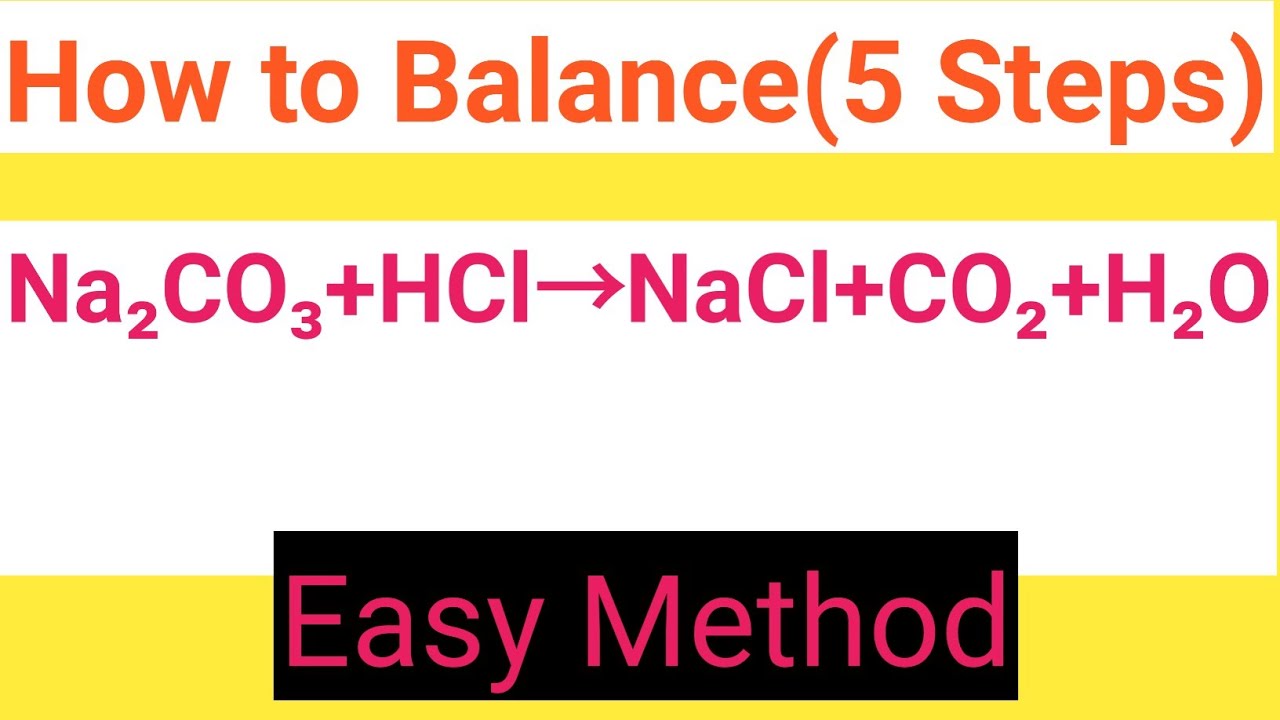
Na2CO3+HCl=NaCl+CO2+H2O Balanced Equation||Sodium carbonate+Hydrochloric acid=Sodium chloride+Carbon - YouTube

Effect of Na2O/SiO2 and K2O/SiO2 mass ratios on the compressive strength of non-silicate metakaolin geopolymeric mortars - IOPscience

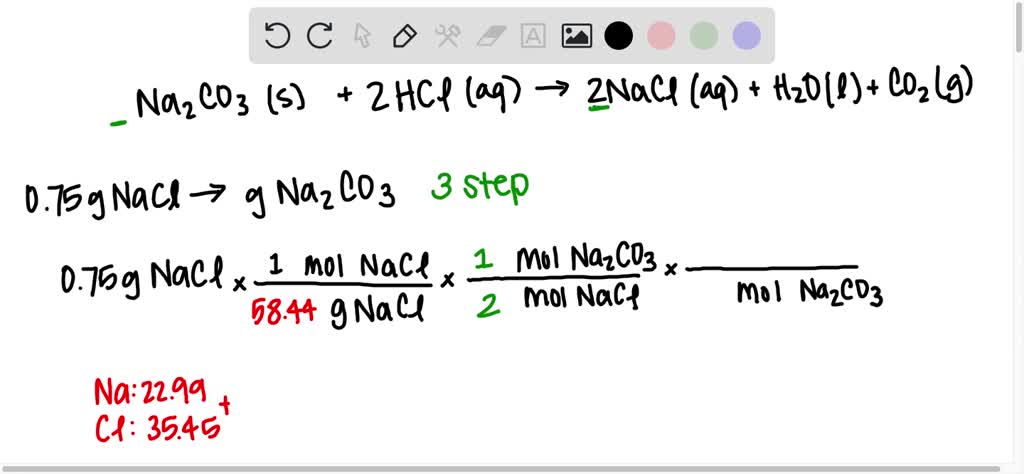
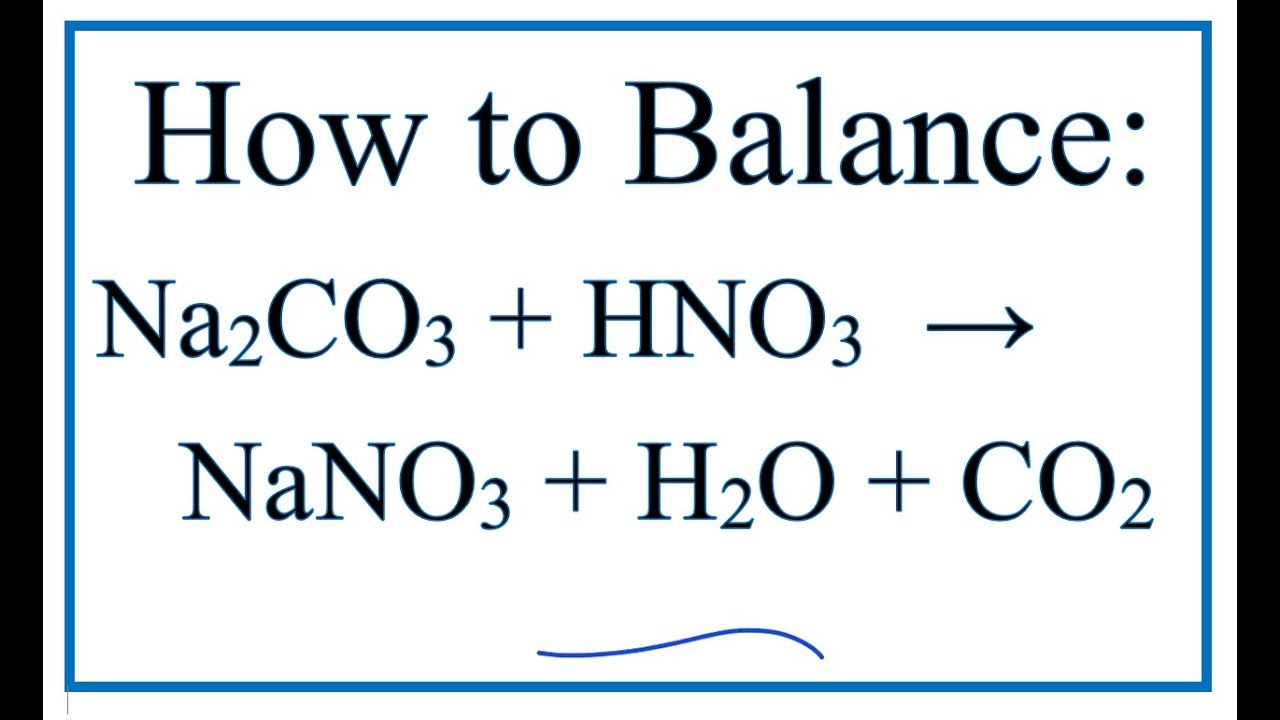
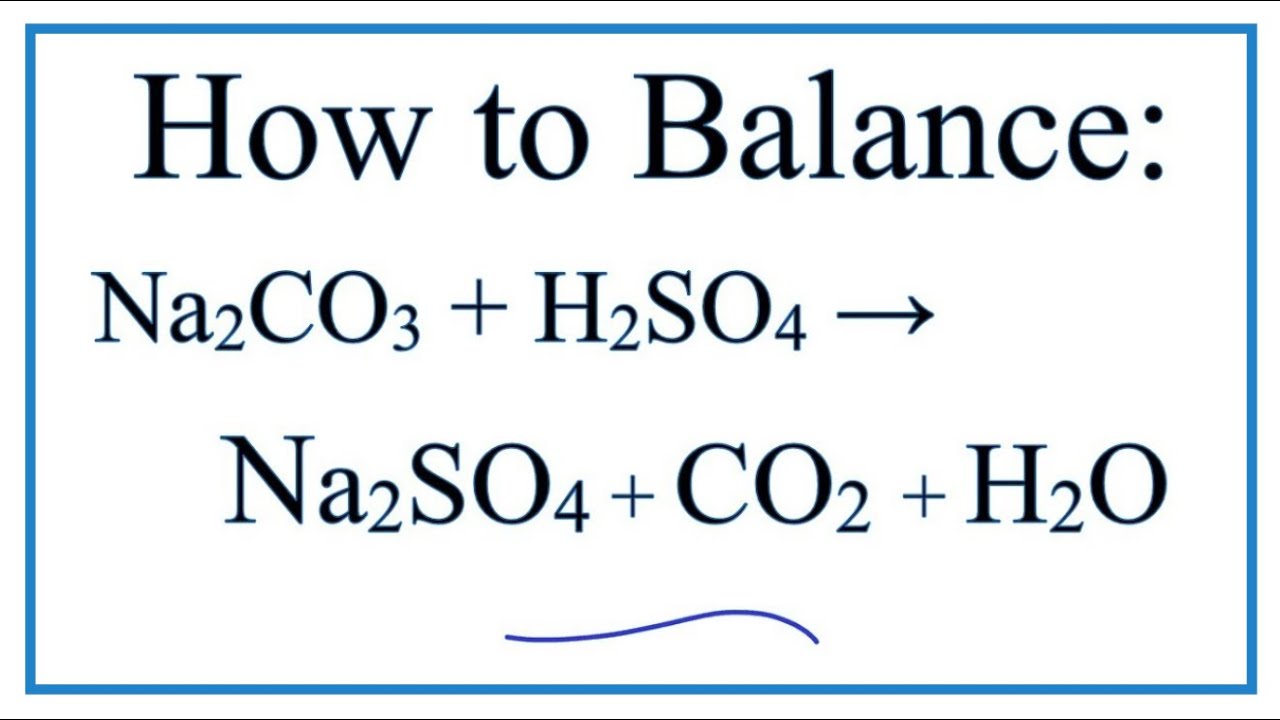
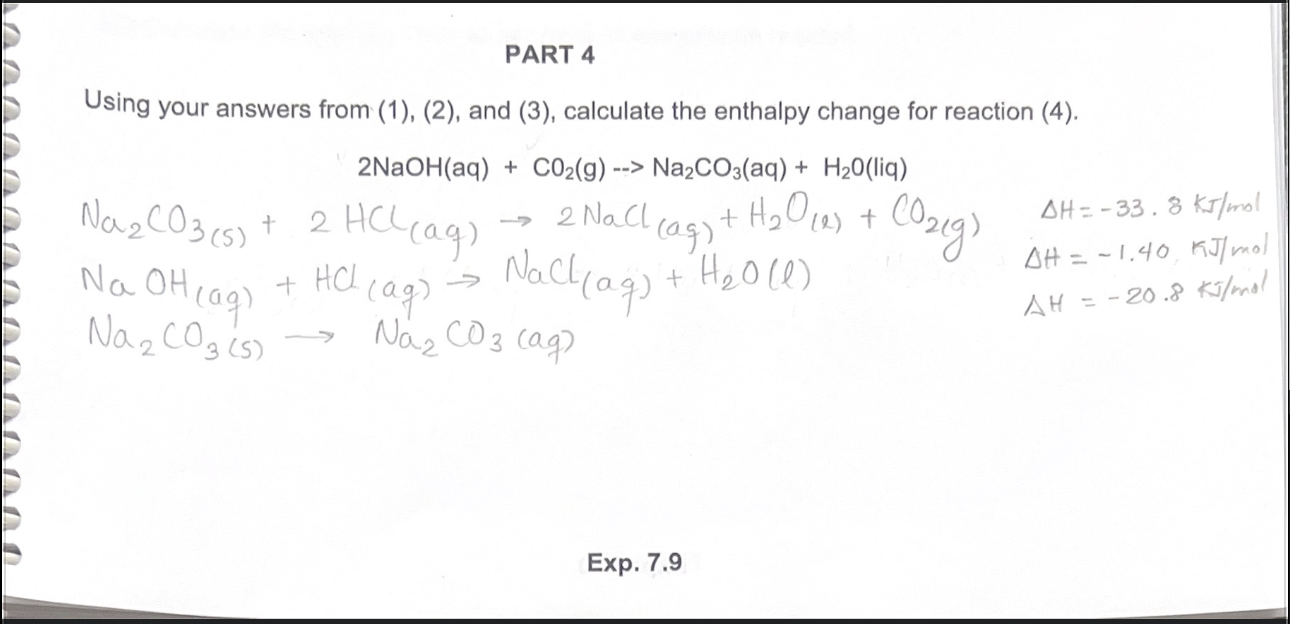
1 Dilute hydrochloric acid reacts with sodium carbonate solution. 2HCl(aq)+ Na2CO2(aq) - brainly.com

Na2CO3+HCl=NaCl+CO2+H2O Balanced Equation||Sodium carbonate+Hydrochloric acid=Sodium chloride+Carbon - YouTube

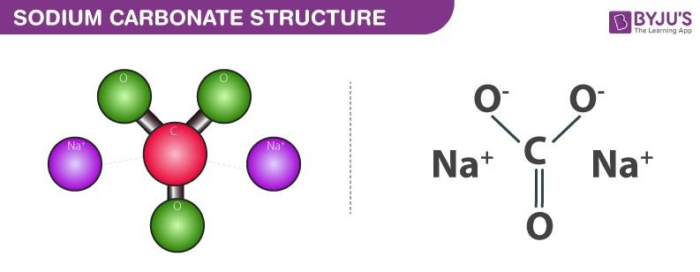
28. State as to why: (a) Aqueous solution of Na,co, is alkaline. (b) BaO is soluble but Baso, is insoluble in water. (1) Draw structure of BeClz (vapour). (m) Complete the following:

Membrane Crystallization of Sodium Carbonate for Carbon Dioxide Recovery: Effect of Impurities on the Crystal Morphology | Crystal Growth & Design

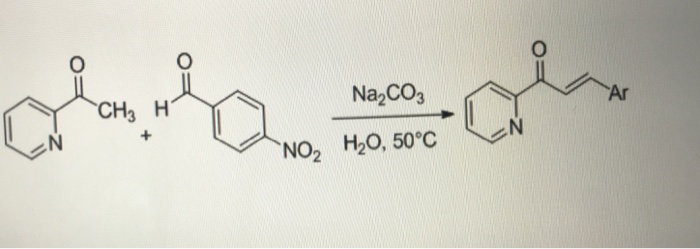
A Strategy for Amide to β-Oxo Ester Transformation via N-Alkenoxypyridinium Salts as the Activator and H2O as the Nucleophile | Organic Letters