K2CrO4 + K2SO3 + H20 -> Cr(OH)3 + K2SO4+ KOH Uzupełnij współczynniki Bilansem jonowo-elektronowym. - Brainly.pl
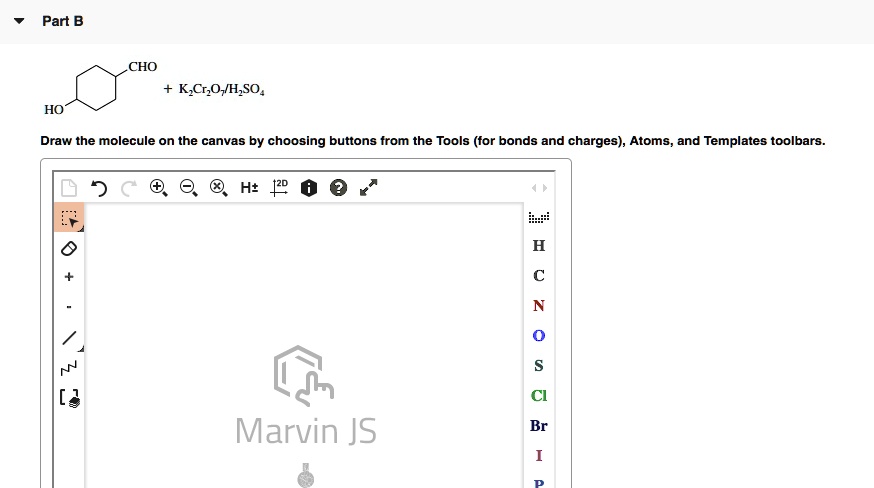
SOLVED: CHO K2CrO4 H2SO4 H2O Draw the molecule on the canvas by choosing buttons from the Tools (for bonds and charges), Atoms, and Templates toolbars. H2O E Marvin JS Br

MJG Cursillo Química Balanceo de ecuaciones Redox (medio básico): CrI3+Cl2+KOH--K2CrO4+KIO3+KCL+H2O - YouTube

The of numerical coefficient that balance the equation K2CrO4 + HCI – K2Cr2O7 + KCI + H20 K2CrO4 + HCI - K2Cr20+ KCI + H20 31fAT HIMA BI Hahalg, ufa g A. 1, 1, 2, 2,1 B. 2, 2, 1, 1, 1 C. 2, 1, 1, 2,1 D. 2, 2, 1, 2,1

A student titrates a 50.0 mL sample of chromic acid, H2CrO4, with 0.200 M KOH (aq). The titration reaches - Brainly.in
Q117) The set of numerical coefficients that balances the equation K2CrO4 + HCl → K2Cr2O7 + kCl + H2O is kerala CEE 2001 d) 2, 2, 1, 2, 1
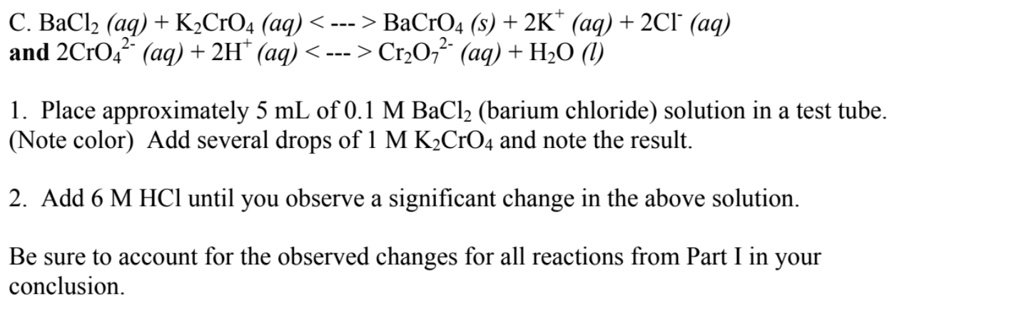
SOLVED: C.BaCl2 (aq) + K2CrO4 (aq) -> BaCrO4 + 2KCl (aq) 2CrO4^2- (aq) + 2H+ (aq) -> Cr2O7^2- (aq) + H2O Place approximately 5 mL of 0.1 M BaCl2 (barium chloride) solution

SOLVED: A standard aqueous solution of potassium chromate (K2CrO4, MW = 194.2 g/mol) is prepared by dissolving 106.3 g of K2CrO4 in 400.0 g of water (H2O, MW = 18.02 g/mol). The

SOLVED: Potassium chromate (K2CrO4) dissolves in acidic solution to generate strongly oxidizing CrO42- ions: K2CrO4(s) + H3O+(aq) â†' 2K+(aq) + HCrO4-(aq) + H2O(l) 2HCrO4-(aq) + CrO42-(aq) + H2O(l) â†' 3H+(aq) + 2Cr3+(aq) +