SOLVED: KMnO4 and H2C2O4 react according to the following balanced equation: 2 KMnO4 + 5 H2C2O4 + 3 H2SO4 –> 2 MnSO4 + 10 CO2 + 8 H2O + K2SO4. If 17.45
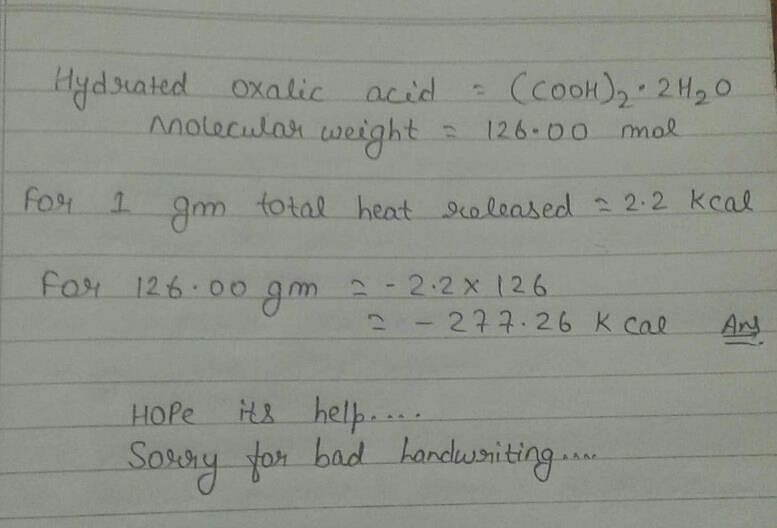
1 g hydrated oxalic acid upon combustion produces 2.2kcal of heat. It's enthalpy of combustion is -277. 2 kcal. Can someone explain how? - EduRev NEET Question
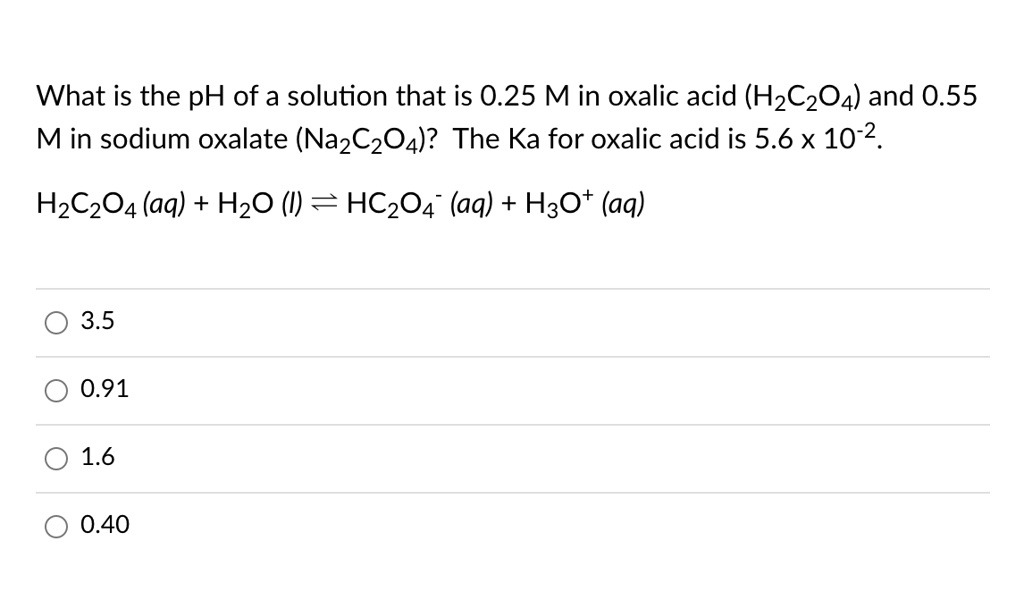
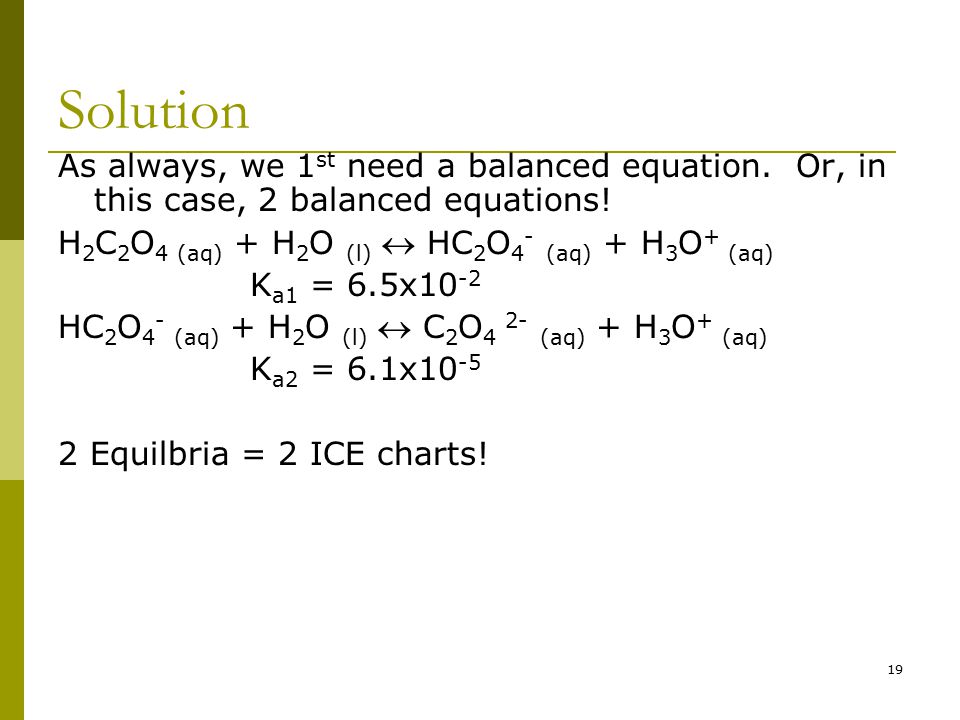
SOLVED: What is the pH of a solution that is 0.25 M in oxalic acid (H2C2O4) and 0.55 M in sodium oxalate (Na2C2O4)? The Ka for oxalic acid is 5.6 x 10^-2.
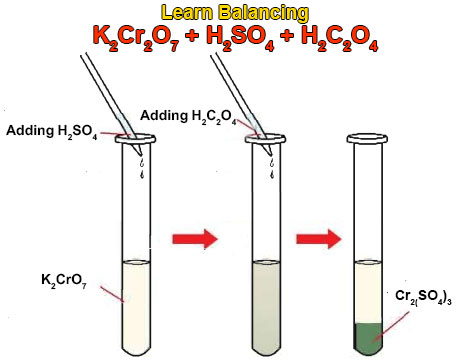
Balance KMnO4 + H2C2O4 + H2SO4 gives rise to K2SO4 + Mnso4 + CO2 + H2O using the alternate method of balancing

NA H2C204 -> gas (A) + gas (B) + liquid (C) (Oxalic acid) Gas (A) burns with a blue flame and is oxidised to gas (B). Gas (B) turns lime water milky.
Balance the following redox equation by half reaction method : H2C2O4(aq) + MnO4^Θ(aq) → CO2(g) + Mn^(2⊕)(aq)(acidic) - Sarthaks eConnect | Largest Online Education Community
1. Commercial vinegar was titrated with NaOH solution to determine the content of acetic acid, HC2H3O2. For 20.0 milliliters of the vinegar, 26.7 milliliters. - ppt video online download

H2C2O4+H2O=C2O4+H3O balance the chemical equation @mydocumentary838. #hashtagvideo #youtube - YouTube