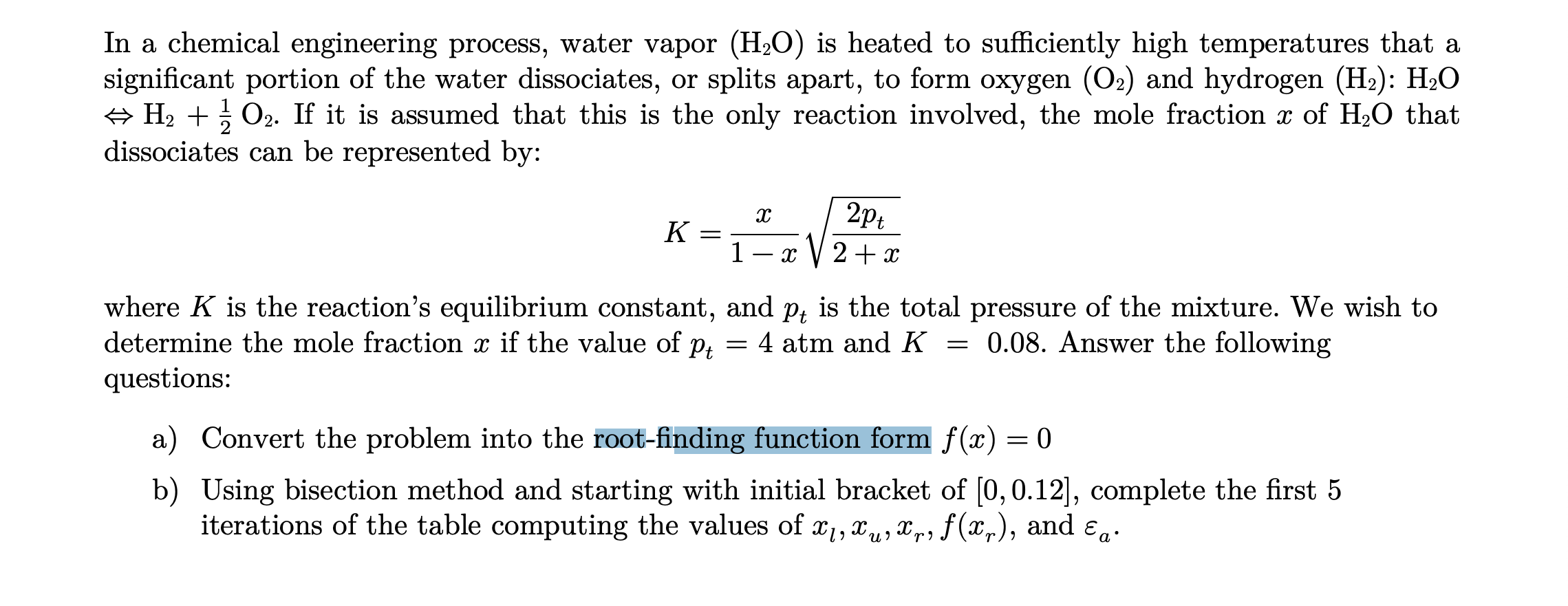
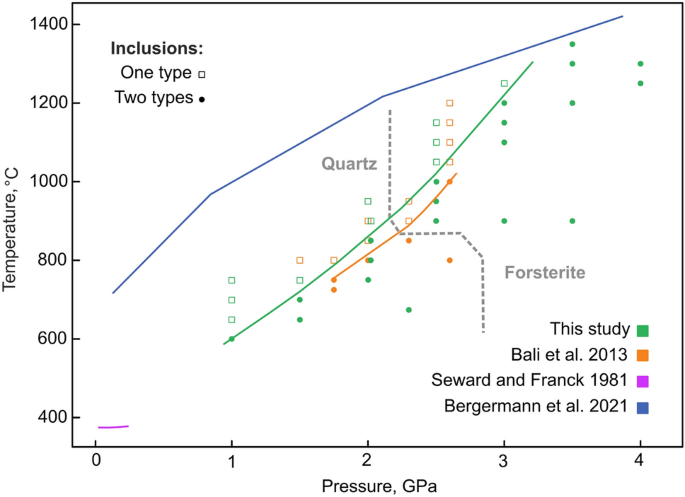
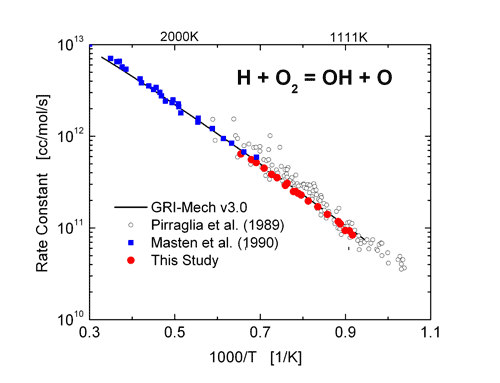
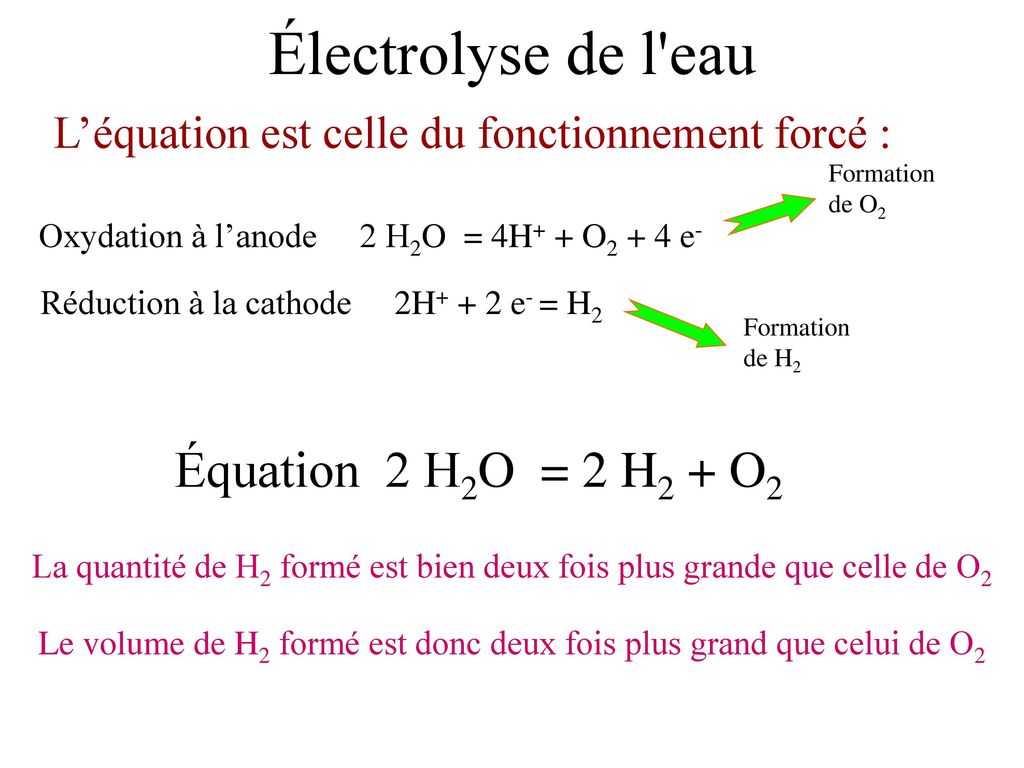
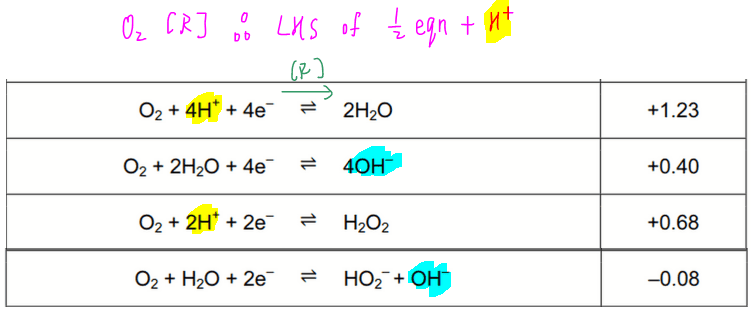![SOLVED: Determine the equilibrium-constant expression for the reaction: CuO(s) + H2(g) ⇌ Cu(l) + H2O(l) ? A. K = [H2O]/[CuO] B. K = [Cu][H2O]/[H2][CuO] C. K = 1/[H2] D. K = [H2]/[Cu] SOLVED: Determine the equilibrium-constant expression for the reaction: CuO(s) + H2(g) ⇌ Cu(l) + H2O(l) ? A. K = [H2O]/[CuO] B. K = [Cu][H2O]/[H2][CuO] C. K = 1/[H2] D. K = [H2]/[Cu]](https://cdn.numerade.com/ask_previews/69a6a069-64fe-4b52-b6aa-a926786b3f81_large.jpg)
SOLVED: Determine the equilibrium-constant expression for the reaction: CuO(s) + H2(g) ⇌ Cu(l) + H2O(l) ? A. K = [H2O]/[CuO] B. K = [Cu][H2O]/[H2][CuO] C. K = 1/[H2] D. K = [H2]/[Cu]

Question⬇️ Does “moles of hydrogen gas” refer to moles of H2? Meaning that 2H2 is 2 moles of hydrogen gas? : r/chemhelp

85. For the reaction, 2NO + 2H2 — N2 + 2H20, the mechanism is given below 2NO=N2O2 N2O2 + H2 - slow > N2O + H2O 4 ) N2O + H2 -
CO2+H2=CO+H2O, 1 mole of CO2 and 2 moles of H2 are placed in a 2L container. At equilibrium, the concentration of CO is 0.28 mol/L. What is the equilibrium constant Kc for

Direct production of H2O2 from H2 and O2 in a biphasic H2O/scCO2 system over a Pd/C catalyst: Optimization of reaction conditions - ScienceDirect

Consider the following reaction at certain temperature: H2O(g)+CO2(g) equilibrium to H2(g)+CO2(g) Some molecules of H2O and CO are placed in a 1.0 L container as shown below. When equilibrium is reached, how
An equilibrium mixture, CO(g) + H2O(g) ⇋ CO2 (g) + H2 (g), present in a vessel of one litre capacity at 1000 K - Sarthaks eConnect | Largest Online Education Community