Mass spectra of RNA 1 (2 μM) in 9:1 H2O/CH3OH with 20 mM CH3COONH4 as... | Download Scientific Diagram

Solubility of H2S in (H2O + CH3COONa) and (H2O + CH3COONH4) from 313 to 393 K and at Pressures up to 10 MPa | Journal of Chemical & Engineering Data
What is meant by hydrolysis ? A solution of CH3COONH4 is neutral. why ? - Sarthaks eConnect | Largest Online Education Community

Green Synthesis of 1,4‐Dihydropyridine Derivative in Water - Isomura - 2018 - ChemistrySelect - Wiley Online Library

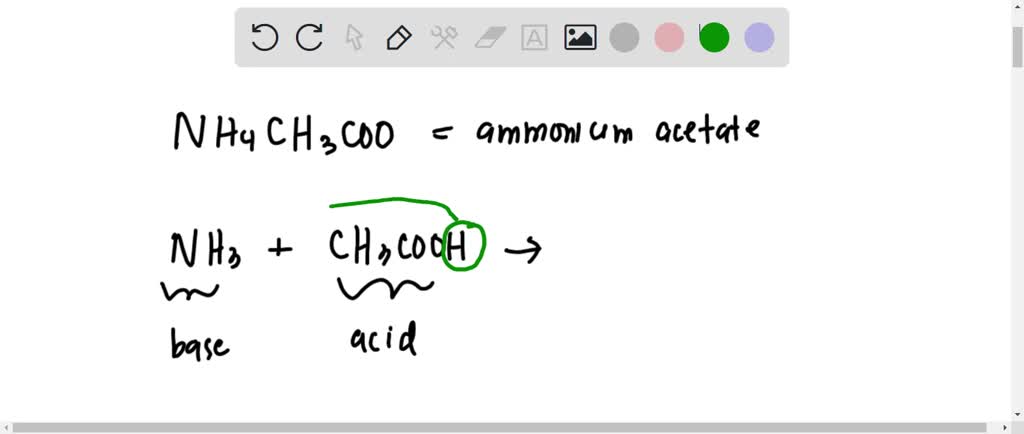
SOLVED: Write equation for the reaction ammonium acetate (CH3COONH4) with water. What will be the pH of its aqueous solution? Calculate the grams of KHP (potassium hydrogen phthalate) needed to react with

SOLVED: 15.84 When ammonium acetate dissolves in water, both the resulting ions undergo proton transfer reactions with water, but the net reaction can be written without using water: NH4+(aq) + CH3COO-(aq) â†'
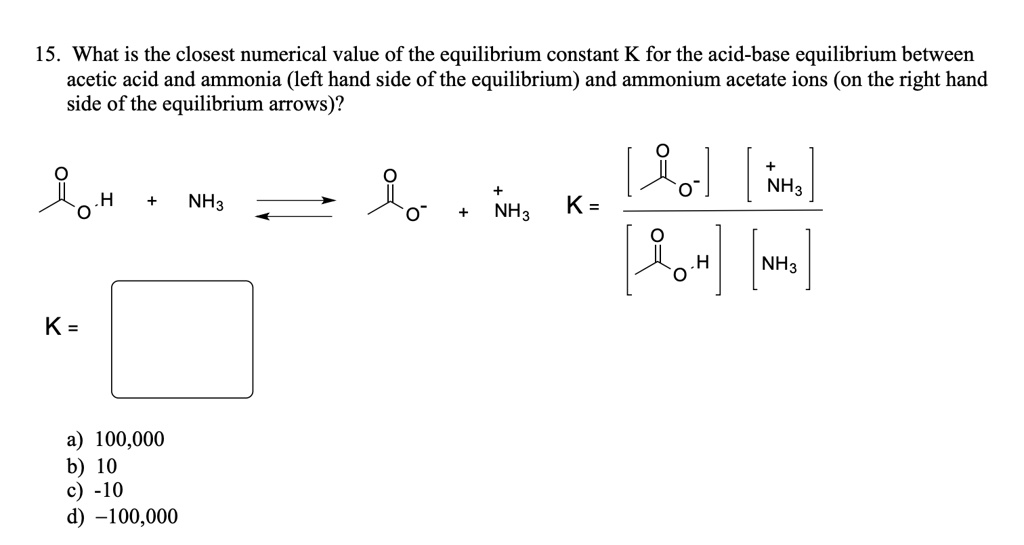
SOLVED: 15. What is the closest numerical value of the equilibrium constant K for the acid-base equilibrium between acetic acid and ammonia (left hand side of the equilibrium) and ammonium acetate ions (

SOLVED: 15.84 When ammonium acetate dissolves in water, both the resulting ions undergo proton transfer reactions with water, but the net reaction can be written without using water: NH4+(aq) + CH3COO-(aq) â†'



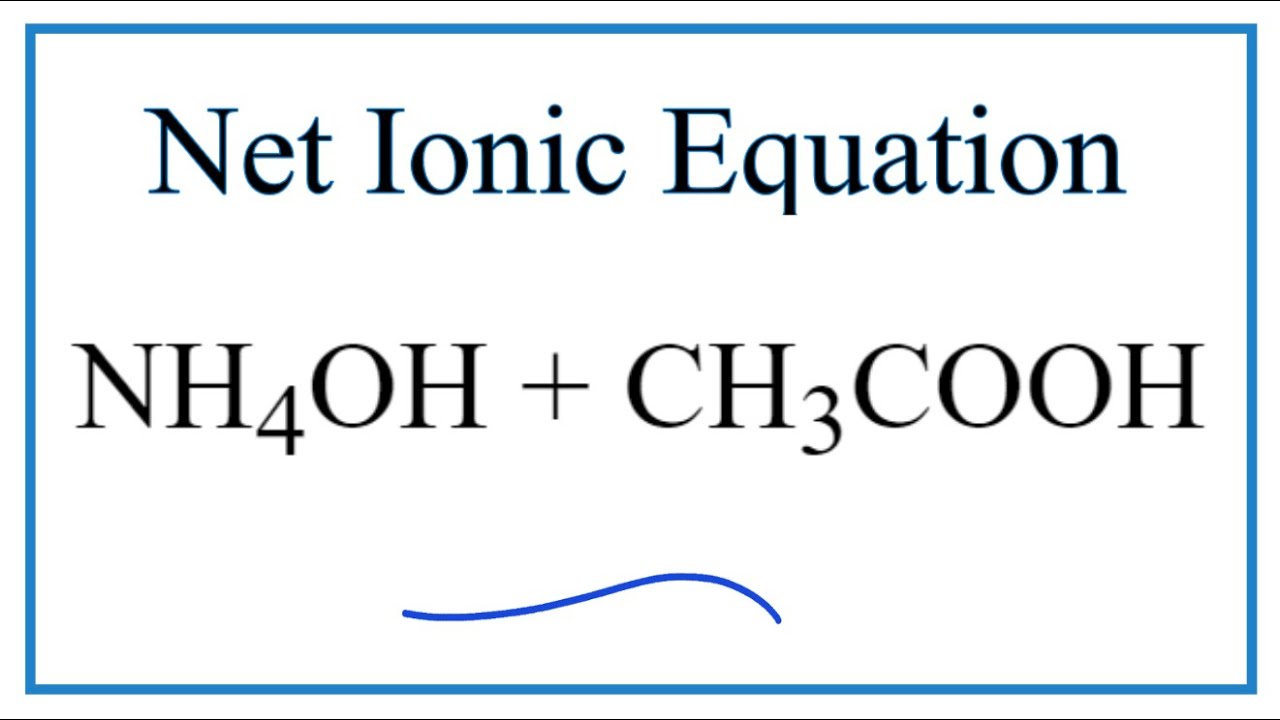
![Welcome to Chem Zipper.com......: [3] CATIONIC AS WELL AS ANIONIC HYDROLYSIS: Welcome to Chem Zipper.com......: [3] CATIONIC AS WELL AS ANIONIC HYDROLYSIS:](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEgL8SQhnjKci8Sd_t_-hwKS6TbUzdVEN36ZVX7jzyaaImcB0R3-pjiQk5I7lhygJIO2mioVSY0269QkoVgPgOTPfaPDfCu_McwLwOKS5MWlvzzjd4qRRjyHV5vZto0d67HeHHMAvK9k81o/s1600/SH28.PNG)


![Welcome to Chem Zipper.com......: [3] CATIONIC AS WELL AS ANIONIC HYDROLYSIS: Welcome to Chem Zipper.com......: [3] CATIONIC AS WELL AS ANIONIC HYDROLYSIS:](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEjJbWjsn-LBqxX4y74j0W2DM5MJz-FPpzBl0Sn2S3KZpbegpZvPApc4JmvPT4I3cwFInFG4BZE3E4x2cYaQys3MI3C6K0Py8tIkE3hWaKVpxk-jBlgrGQPHBLUZ4XcVBrz1C1IGUAtPybQ/s1600/SH21.PNG)