16. In a chemical reaction, caco3+2hcl= cacl2 +co2+h2o. 25ml hcl and 0.75M Calculate the amount of caco3
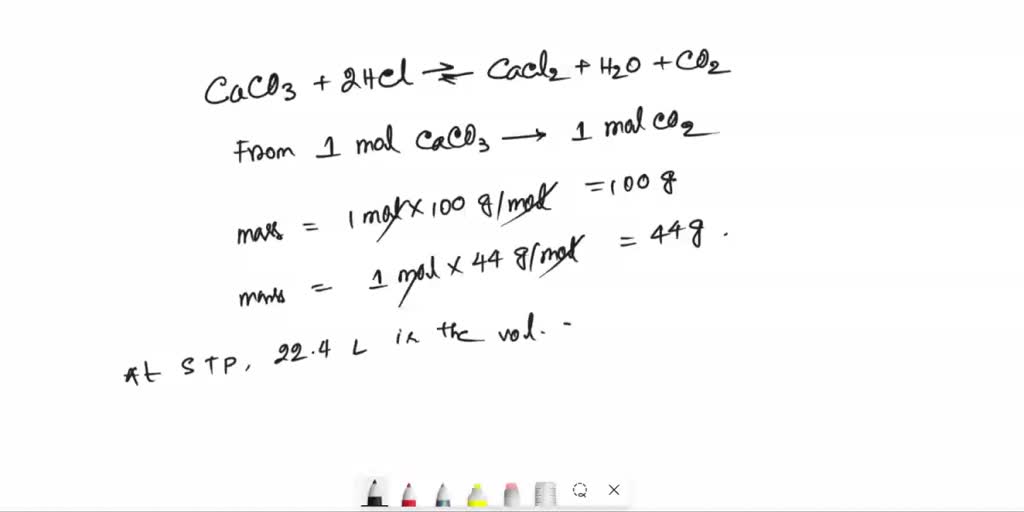
SOLVED: When the calcium carbonate is reacted with an excess amount of hydrochloric acid, how much CaCO3 is required to produce 11.2 liters of CO2 at STP ? (Given molar masses :

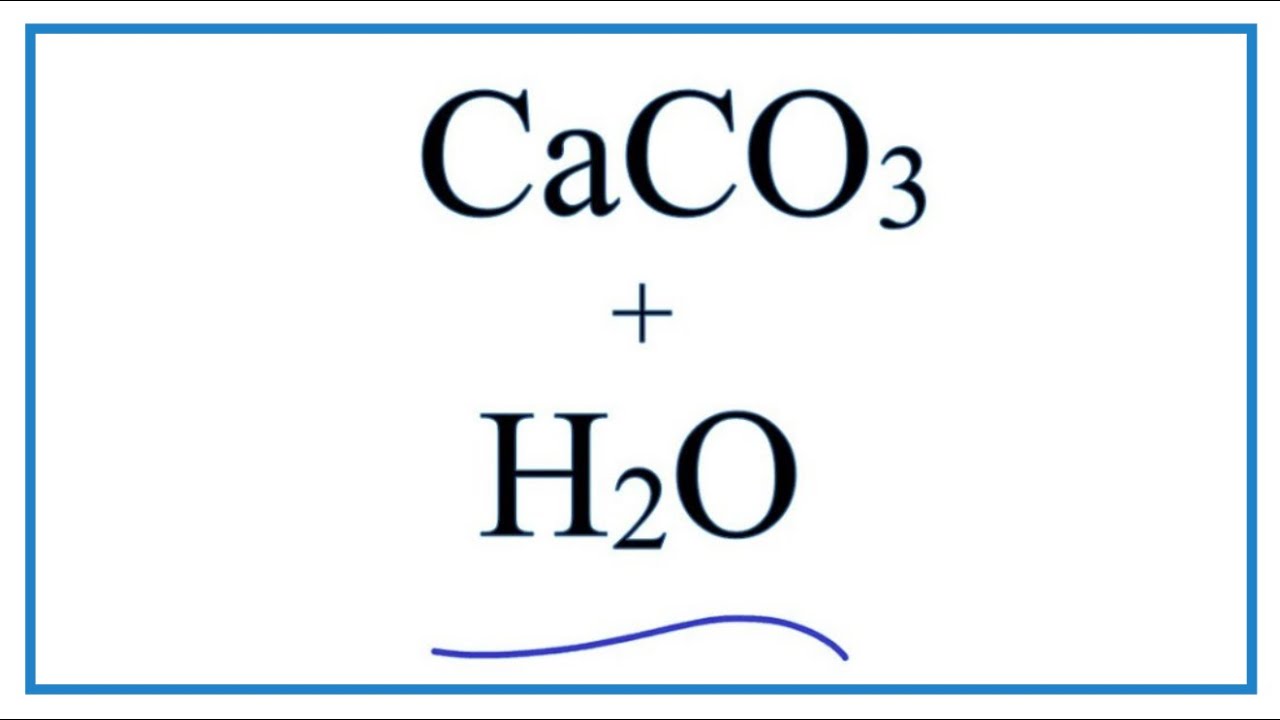
Section - B 15. Complete and balance the following chemical equations : (1) NaOH(aq) + Zne) → (ii) CaCO3(e) + H2O + CO2(2)→ (iii) HCl(aq) + H20) ▻ OR
The mass of CaCO3 required to react completely with 20 mL of 1.0 M HCL as per the reaction CaCO3+2HCl–>CaCl2+CO2+H2O

SOLVED: CaCO3(s) - CaO(s) + CO2(g) ΔH = 175 kJ Ca(OH)2(s) - H2O(l) + CaO(s) ΔH = 67 kJ Ca(OH)2(s) + 2 HCl(g) â†' CaCl2(s) + 2 H2O(l) ΔH = -198 kJ

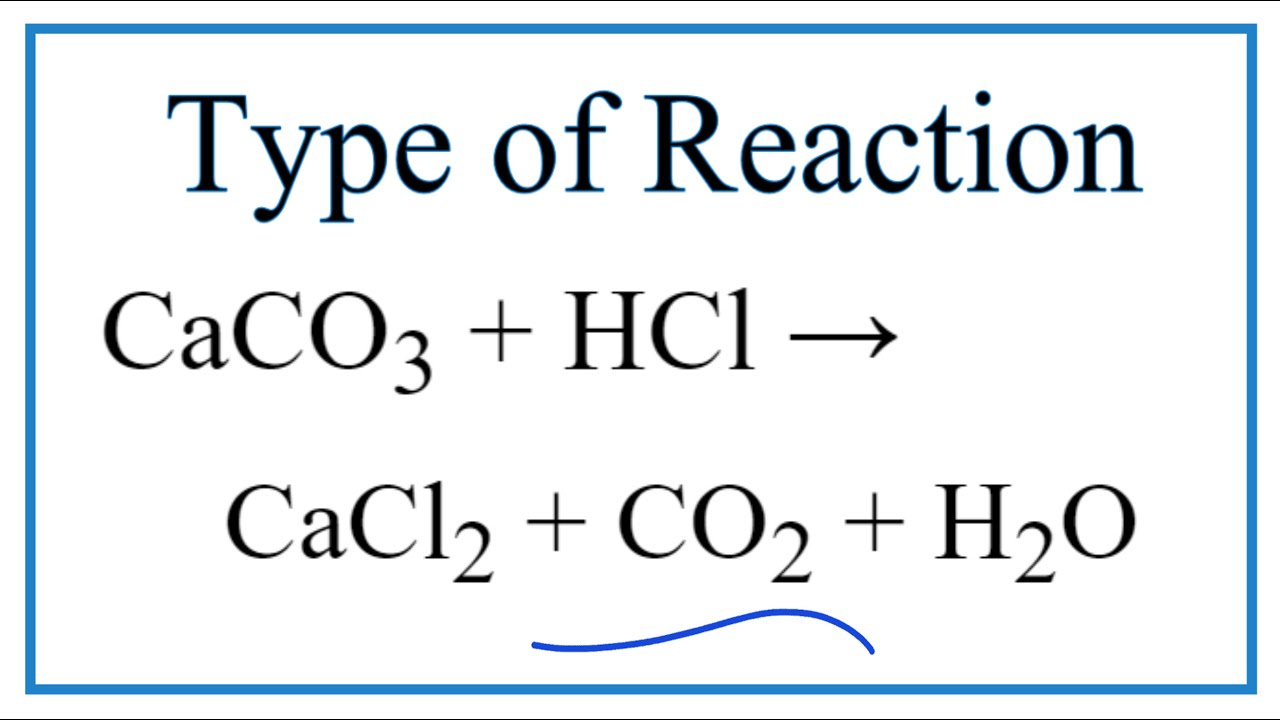
HCl+CaCO3=CaCl2+H2O+CO2 balance the chemical equation @mydocumentary838. hcl+caco3=cacl2+h2o+co2 - YouTube

☆ Balance the chemical Reaction -1)CaCo3 + H₂O + CO2------- Ca(HCO3)22) NaOH + HCl ------ NaCl + H₂O 3) NaCl - Brainly.in

How to Balance CaCO3 + HCl = CaCl2 + CO2 + H2O | How to Balance CaCO3 + HCl = CaCl2 + CO2 + H2O Hey there! Are you struggling with balancing

Consider the reaction : Caco, + H2SO4 → CaSO4 + H2O + CO2 What will be the percent purity of Caco, 200 g Caco, produces 22.4 L Co, STP in excess of H2SO,? (1) 25% (2) 50% (3) 75% (4) 100%












![Explain the reaction.CaCO3+2HCl⟶CaCl2+H2O+CO2[g] Explain the reaction.CaCO3+2HCl⟶CaCl2+H2O+CO2[g]](https://search-static.byjusweb.com/question-images/toppr_ext/questions/1352729_1287783_ans_adc66c29654147fb965de67e86f7f9a2.jpeg)


