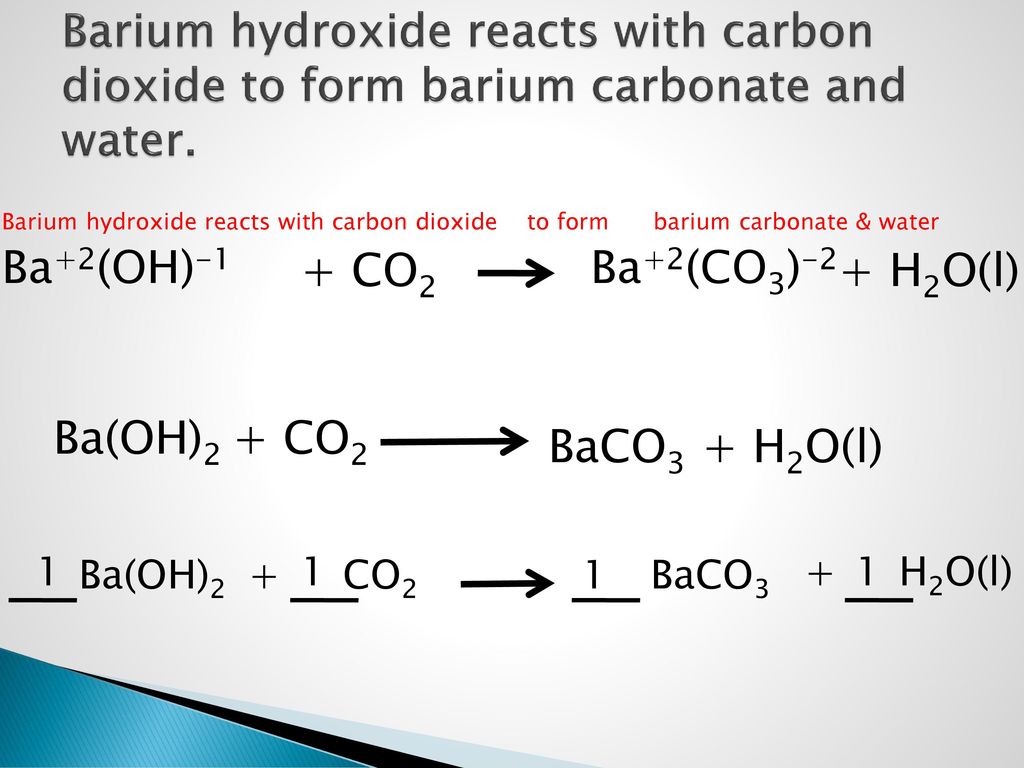
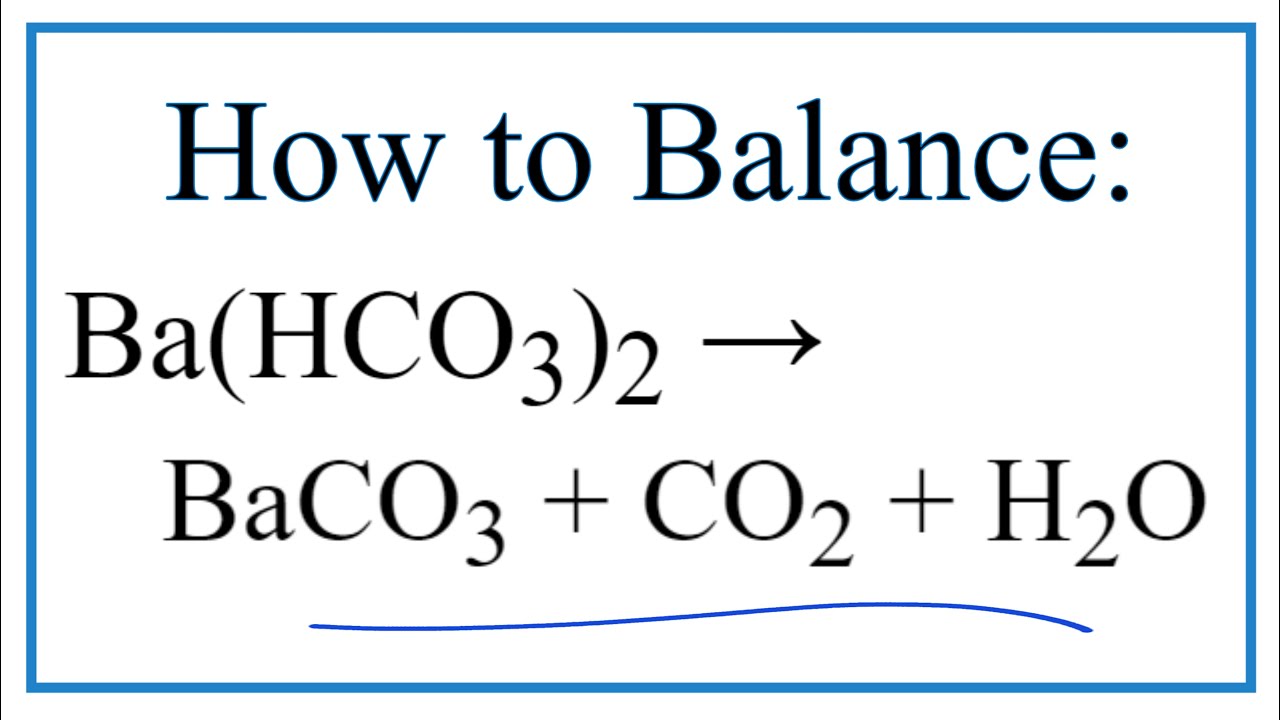
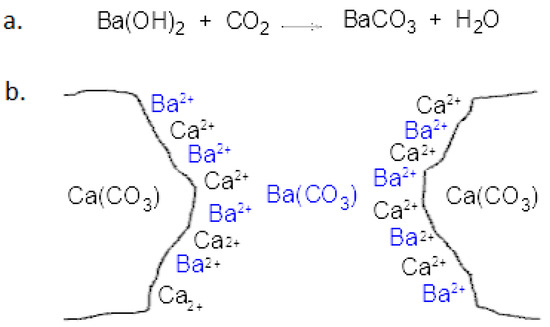
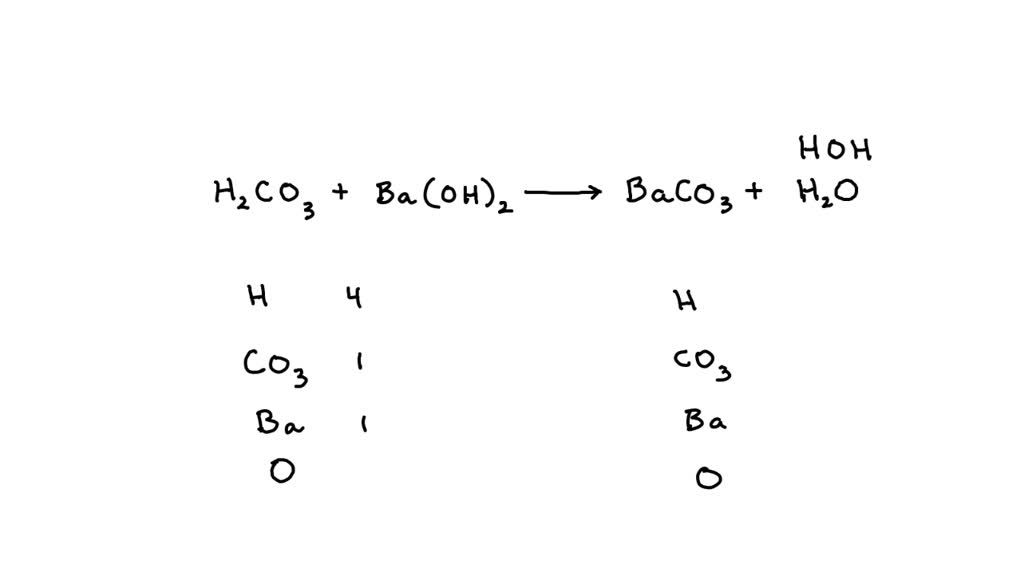24.Write equilibrium constant expression the following reac (i) BaCO3 (8) ---------- BaO(s) + CO2 (g).
Heritage | Free Full-Text | Characterization of Barium Hydroxide Used as Consolidating Agent for Monumental Surfaces in Venice

Solve 53 sum 200 m' solution molarity of tho volumo ot CO, at S T P on heat'0 9 (2) - Chemistry - - 12898155 | Meritnation.com

SOLVED: Equation: Ba(OH)2(aq) CO2(g) BaCO3(s) H2O(l) Total ionic: Ba2+(aq) + 2OH-(aq) + CO2(g) â†' BaCO3(s) + H2O(l) Net ionic: Ba2+(aq) + CO2(g) â†' BaCO3(s) + H2O(l)
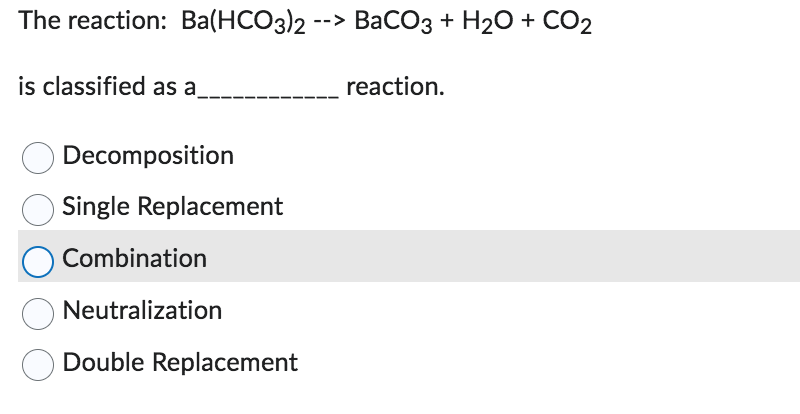
Solved: The chemical equation here describes a reaction between barium carbonate and nitric acid. [algebra]

PPT - What is the difference between a chemical reaction and physical change? PowerPoint Presentation - ID:5813241

I1. LUYEΝ ΤΑΡ: Bài tập 1: Hoàn thành các phản ứng hóa học sau: a. SO, + ? → H2SO3 b. ? + H2O → KOH C. ? + ? СаСОз d. CO2 + ? → BaCO3 + H2O e. ? + H2SO4 f.
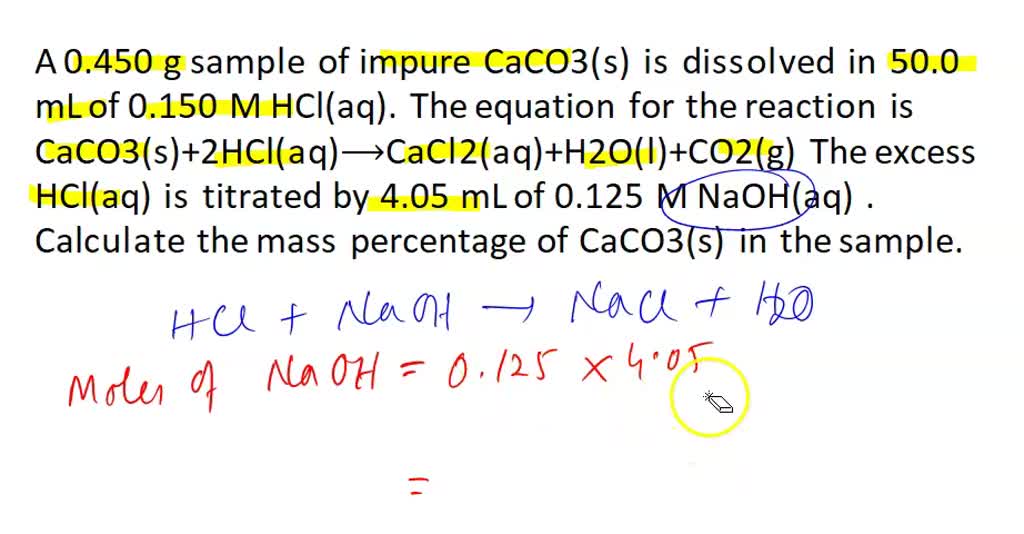
SOLVED: (b) The Ksp of barium carbonate, BaCO3, is 2.58×10^(-9). Calculate the molar solubility, S, of this compound. S= (c) A 0.450 g sample of impure CaCO3(s) is dissolved in 50.0 mL

a. SO2 + ? → H2SO3 b. ? +H2O → KOH c. ? + ?→ CaCO3 d. CO2 + ? → BaCO3 + H2O e. ? + H2S04 → MgSO4 + H2O f. Fe + HCl– → ? + ? g. Al203 + H2SO4 → ? + H2O h. N





![ANSWERED] BaCO3 + 2HNO3 → Ba(NO3)2 + CO₂ + H₂O What ... - Organic Chemistry - Kunduz ANSWERED] BaCO3 + 2HNO3 → Ba(NO3)2 + CO₂ + H₂O What ... - Organic Chemistry - Kunduz](https://media.kunduz.com/media/answer/raw/20220422054128757632-4413054.jpg?type=wm)